- Home
- About Us
- Join/Renew
- Member Benefits
- Member Pages
- Log In
- Help
- Museum Store
I started looking into repairing my fuel gauge and there are many many postings online regarding the dye used by Classics and Exotics fading very quickly. Has anyone had more recent experience with their repair kit and whether or not the dye fade is still an issue with that kit.
Thank you,
Mark
All the new dye has a shorter life than the old stuff…………..I have nine cars that use it, and they fade at different rates in different gauges in each car…….go figure…..
Thank you Ed, I asked them about it and they claim that they have absolutely no issues with the dyes fading, but a simple google search brings up far to many comments to the contrary. That said, I can get that same quality liquid for less through a Ford dealer than them and they have the same problems – I am sure it’s all coming from the same sources.
If anyone knows of a product that isn’t fading I would be grateful for that referral.
I find the hydrostatic gauge interesting, but I am beginning to lean toward converting the ‘31 to an electric gauge after reading about all the issues.
The K-S dyes WON’T FADE if you keep the vial in a Desk Drawer.
The easy way to have a K-S fuel gauge that “appears to work” is to cut a piece of RED WD40 tube and drop it into the K-S gauge head.
I have K-S that either does not work, or the dye has faded.
I don’t worry about my fuel level, as I just fuel up every day I drive my Pierce.
Also, wooden dowels make great fuel level sticks because unlike most yard-sticks, they don’t have any varnish / polyurethane on them.
per a previous thread: I did an experiment a few years ago trying to determine how fast the K-S fluid would lose its color and if the fluid from different vendors lasted longer. I purchased new K-S fluid samples from both vendors (Ford and Classic) and put them in glass dropper tubes and set them directly in the Nevada sun for the summer. Some had UV protective glass in front of them. None of them had turned color by the end of the summer, and to date still haven’t turned sitting around in my garage.
Oddly, however, the fluid I had in my K-S fuel gauge for the test of my system was bright red for the test but after a couple of months in the garage without direct sunlight it suddenly turned light amber in the space of a week or two. That fluid was from a vial I had bought over 20 years before and had been carefully kept in its black light blocking packaging in a closed box the entire time until put in the gauge for the test.
I think it is a combination of age and light, not just the light exposure alone.
The best chance is buy fresh fluid and it should last a few years. I may put my test samples back in the sun for the summer to extend the test.
Jim

Thank you Jim, that’s the article that kick started my searching out if anyone has made any improvement in the quality of the dyes used. There is also a thread somewhere about adding more dye to the faded solution that went back around 10-12 years ago on a Ford forum but I found several negative threads that were posted well after those. I’m going to try to get mine to work, and if I have to I’ll put a read bead that will float on the fluid so I can see it after It fades out…
Thank you all for the comments!
Hi Mark, when you find a red or black or ? color bead that will go into the glass tube and float on the fluid, buy me a few too!!
My ’32’s and ’33 all have faded fluid. I added fresh to my ’32 Conv. Coupe last year, and it has pretty much faded away.
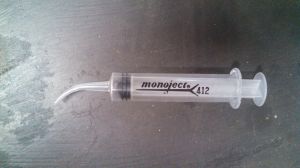
A suggestion for adding the fluid: If you search for ‘curved-tip syringe’ you should find the lubrication syringes that have a 1/2″ radius curved plastic tip on the end, tapers to a point. much like a dog’s toe-nail.
pull in a small amount of the red fluid, then look under the dash and find the top of the gauge, insert the small curved tip carefully into the top of the glass tube, then hold the syringe in place and hold a mirror to see the gauge. add fluid until it reaches the bottom of the glass tube.
Normally an eyedropper is suggested, but with as corrosive as the fluid is, This syringe makes it much easier and safer to add fluid.
Hope this helps.
Greg Long
Thank you again Greg
James,
Were your test samples also exposed to the open air, as your photo makes them appear to be contained in upside down, and therefore, sealed bottles.
I do not believe that the K-S gauge is sealed at the top of the head unit.
If so, perhaps exposure to air is also a fading factor.
Peter
No they aren’t sealed, the tubes are open at the top just like the K-S fuel gauge. The tubes were made from glass eyedropper tubes sealed at the bottom by melting the glass tip with a torch.They are in a frame that keeps rain from getting in when left outside.
The temp gauge is sealed I believe.
I have a total of 3 samples each from the two vendors and the 3 pairs are behind regular glass, 99% UV filtered glass and a UV filtered computer eyeglass lens. the idea was to see if UV filtering would extend the life of the color, but to date none have faded so no way to tell. If the weather ever gets to being like summer I will put them out in the sun again.
The colored bead inside is a great idea but the K-S fluid is some high density chemical that can be corrosive, so not sure what material – including color- a ball could be made from and survive. Has anyone done this?
Jim
“not sure what material – including color- a ball could be made from and survive””
Sounds like you need to do another science experiment 
Robert”
Take the WD40 tube, seal one end, cut to short length and use it as a float??
Not a bad idea Bill, I might try that experiment.
Here is a photo of the lubrication syringe I use to add fluid to the gas gauge glass tube.
Greg Long
I have asked my chemistry pHD nephew if he has any ideas. Meanwhile the fluid is apparently acetylene tetrabromide (1,1,2,2-tetrabromoethane) and has a specific gravity of 2.96 (2.96 denser than water, about the same as aluminum). I am not 100% certain of the identity as it is supposed to have a pretty strong smell like camphor but I haven’t noticed that, It is referenced on several sites as K-S fluid.
The following doesn’t look promising for floating plastic balls:
From MSDS: ACETYLENE TETRABROMIDE
Incompatibility: Reacts with chemically active metals or strong caustics. In the presence of steam,contact with hot iron, aluminum, and zinc may cause formation of toxic vapors.
Softens or destroys most plastics and rubbers.
It is considered pretty toxic. Here are some warnings from chemicalbook.com:
H302 Harmful if swallowed Acute toxicity,oral
H315 Causes skin irritation Skin corrosion/irritation
H319 Causes serious eye irritation Serious eye damage/eye irritation
H330 Fatal if inhaled Acute toxicity,inhalation
H335 May cause respiratory irritation Specific target organ toxicity, single exposure;Respiratory tract irritation
be careful out there!
Jim
Checking chemical compatibility charts indicates that polyethlene is not recommended for long term exposure (>1 year) to acetylene tetrabromide. PE may be the plastic used for WD-40 tubes. There are two compounds that are listed as compatible: PTFE (Teflon) and Viton.
PTFE is a bit less dense than acetylene tetrabromide, so it should float. I found a red PTFE ball as small as 1/8â€, but my K-S glass tube is ~1/8†so I think that ball would stick in the tube. I found natural white PTFE balls down to 1/16†dia. Perhaps one solution would be to paint a red stripe behind the glass tube and use the white PTFE ball to indicate level.
I also found 3/32 black Viton balls that should float also.
It probably wouldn’t be easy when in the car, but a ball could theoretically be dropped into the glass tube by removing the little cover above the tube. Would need some sort of clever tool to make it happen.
Jim
Take care not to spill or drip the fluid on the face of the gauge as it will destroy it.
H330 and H335.
Guess you have to drive with the windows open, don’t breath, have an airline respirator or some combination thereof.
Since the fluid hardly evaporates at all at room temperature -it can sit for years in an open K-S gauge for years – I am guessing the concentrations emanating from our cars is miniscule, which explains why we haven’t dropped dead handling it. I also assume that the low vapor pressure explains why I don’t smell it. Heating it up is probably where things would get nasty. It does seem skin and eye protection is warranted.
My nephew has responded and thinks the brass and copper of the reservoir and capillary tube is likely a part of the problem, it may be a chemical reaction that gets started generating copper bromide that would explain the sudden loss of color in the dye. Some gunk or particulates in the fluid would be an indication of this.
I need to add some copper bits to my exposure experiments.
My nephew is experimenting with some highly stable and very fluorescent day-glo green dyes if anyone is looking for something different!
Jim
Well, I ran away for the holiday and came back to quite the conversation…
I have some reddish color Viton washers somewhere that were kicking around my desk when I retired that I might try making a bead from and see if I can get it to fit the glass. A very interesting subject, please keep the comments coming!
Thank you for the information,
Mark
