- Home
- About Us
- Join/Renew
- Member Benefits
- Member Pages
- Log In
- Help
- Museum Store
It is pretty hard to tell if the available clock arm pullers are the right scale for this particular clock, with flanges thin enough to get in between the minute and hour hands, and then strong enough to force them off. There is enough of the shaft sitting proud of the minute hand to make a miniature puller with a screw to push directly on the shaft instead of just trying to pull it off as shown. I am going to do that today. Thanks, Jim
Interesting idea, just brushing the whole eraser across the surface instead of trying to meticulously keep on the raised surface with a fine tip?
Thanks, Jim
Well, certainly is a complicated subject, with lots of opinions on the internet. Electrical not my thing. Apparently the DE-OX is a dielectric grease that one would think was an insulator but apparently does not increase resistance in a connection but rather keeps it from corroding, so I am guessing it won’t improve cleaned terminals initially but keep them from deteriorating over time and exposure. It is used on a lot of connections including battery terminals to keep them from oxidizing.
Many articles saying conductive greases aren’t, that they don’t actually improve connections and can cause corrosion problems if the wrong filler (carbon, silver, zinc) is used. Never the less there seem to be plenty of them out there.
The Stabilant 22 itself looks like the very amazing magic but expensive bullet.
It claims it can replicate the connection of a soldered joint, while amazingly not conduct electricity outside. Looks like it is a pretty big deal in the world of computers and audiophiles. I got very confused because the SL-5 was listed as a silicone dielectric grease for protecting spark plug connections but also shows up when searching Stabilant 22. It appears Standard Ignition products sells the SL-5 as a kit including the silicone dielectric and a tiny bottle of Stabilant 22 as well.
Jim
Thanks, I’ll check them out. Jim
Thanks for responding Bob, it looks like the Deox is primarily for preventing corrosion more than improving conductivity in the connection. I am looking at the following product:
MG Chemicals Carbon Conductive Assembly Paste
it claims the following:
Improves electrical connections between irregular surfaces, loose or vibrating parts and small gaps. Does not separate or bleed at high temperatures. Contains special corrosion inhibiting compounds
Low Volume Resistivity: 23 ohms·cm
Volume Conductivity: 0. 04 S/cm
Surface Resistivity: 271 ohms·cm
Surface Conductivity: 0. 0037 S/sq.
Improves Automotive Connections
Bob, sounds like a neat solution to do it yourself, did you take any photos of the setup?
David, thanks for your comments, and thanks of course for your great help on my seat structure, springs as well as the article on the original springs. It never would have occurred to me that there were different coil characteristics to worry about without it!
Jim
Peter, I don’t know either, Brooks mentions it further up in the thread.
How can one access the Schwabacker-Fry files? I have a friend who has been trying to learn if the legend that his semi custom Packard was owned by a PBD (Pretty Big Deal) in Hollywood or the studio.
Thanks, Jim
Well, lets add soem confusion. Looks like maybe they aren’t the same despite what the parts book seems to say. Here is a picture of my repro levers from Blonder assembled on my 1935 845, may be moot for your 836. The plastic cover and horn center button are original – as a far as I know. I might have the levers in the wrong positions (light vs throttle) – they aren’t clamped at the bottom light switch assembly yet. At any rate, one lever is part of the chromed outer ring cup and the other is just inside the outer ring. The V-12 in Greg’s picture appear to attach closer to the center. Jim

Bill I assume you have checked with Dave Murray, I bought repros of the levers from Irv Blonder many moons ago, perhaps Dave would still have some? From the parts book it looks like they were common for all ’33 to ’35, likely ’36-38 as well?
If Ed had them machined years ago, it would be interesting to make a CAD model and see what a it would cost to have them CNC machined today. The costs have come way down for small run parts using CAD-CAM.
The Rickenbacker water pump may be a good candidate for machined CAD-CAM as well.
Jim
I contacted a member I know in CCCA whoi forwarded to Jay Quail. Here is the repsonse:
Hi Jim and Paul,
This an obvious oversight. In an attempt to streamline the list we combined some classes and years and it appears the 1926- 38 PA’s were omitted somehow.
We will make the correction in print in our 2020 handbook, and I will make the correction online in the next 48 hours.
Sorry about the omission.
Jay Quail
Executive Director
CCCA
Yes, when I joined CCCA ca 1981 there was no doubt that the club’s original interest was high end luxury cars built between 1925 and 1939. Within its original year limits all Pierce-Arrows were considered Classics. All Senior Packards in that original date range were but Junior Packards were not, unless on a case by case basis they had exemplary custom coachwork. Likewise I believe even Ford’s were accepted if they had exemplary custom coachwork installed when new. A few years before Lincoln Continentals to 1948 were given Classic status, as it was hard to argue that a 1941 Continental was a Classic but a 1948 that was the same car except for cosmetics wasn’t. From there a number of lower priced late 1930’s and 1940’s Cadillacs were added because it was hard to argue that they were substantially different than the more expensive models that were given Classic status. I know many old car people assumed that the years would be extended into the 1950’s as the cars got older, so it was surprise to me to learn they went the other way.
I would think the mistake was unintentional printing error.
On this site I am never sure when the snide comments about Packard are to be taken at face value and when they are just tongue-in-cheek joking. I have no doubt that if I showed up at a western region Packard tour with my Pierce I would be welcome and the car would be appreciated. On the other hand at this point I don’t feel like I would ever dare to show up at a Pierce meet with my Packard.
Would this also be the best stuff for vacuum windshield wipers?
Greg,
To try and untangle what is going on first we need to understand the basic properties of the fuel and air under ideal conditions. Then we can use that starting point to try and sort out the huge number of variables that happens in a single cylinder of an actual engine, then we compound the problem with multiple cylinders, carburetor and intake manifold.
I get the impression that general observations and tests of a few specific engines are being used to try and understand the fundamental properties of the fuels, rather than first understanding the properties of the fuels and then use that knowledge to try and understand what is happening in the specific engines. A famous example of the difference is James Watt. Steam engines had been pumping the mines of Britain for 60 years when Watt used the fundamental properties of water’s latent and sensible heat measured by he and Boulton to understand why Newcomen engines were wasting 95% of the heat energy available from the coal to heat the water and generate steam. He used this understanding to invent the principle of external condensing and doubled fuel efficiency -wasting only 90% of the coal’s available heat energy. It sparked the industrial revolution.
For 40 years as part of my career, I did thermodynamic modeling of engines, primarily aircraft gas turbines (fanjets, turbojets and turboprops) but also piston engines. This to predict the thrust and fuel burn for an engine flying at 55,000 feet Mach 1.5 (for example) from data originating from an engine run motionless in a test cell on the ground. Sounds complicated but gas turbines are not nearly as complicated as antique piston engines! My first foray into engine research was trying to do a computer model of the products of combustion in a single cylinder piston engine and then testing the engine on a dyno. The model of combustion had to iterate the equations for 14 separate chemical reactions with 14 unknowns, and shall we say, taxed the IBM 360 mainframe I was using.
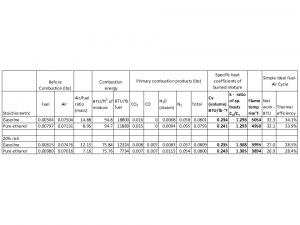
The combustion energy comes from what is known as a bomb calorimeter. It is a fixed volume container of fuel and excess air mixture placed in a water bath and the rise in the bath temperature is used to measure heat of combustion. It is the standard method for measurement of basic energy from combustion. That may seem like a very esoteric method that doesn’t relate to what happens in a real engine, but how can one derive the characteristics of a fuel by looking at dynamometer data from a specific multi-cylinder piston engine with unknown mixture distribution, big cooling losses, internal engine friction, etc etc? Your business aircraft engine performance was based on assuming a standard 18,400 BTU/lb for jet fuel weighing 6.7 lbs/gallon derived from these standardized tests. A remarkable thing about hydrocarbon fuels is how little difference there is in basic heat of combustion per pound from the lightest methane to bunker “C†crude used in Diesels.
The flame temperature rise I put in the table is theoretical as it has the simplifying assumption that the piston comes to a complete stop while the perfect mixture is burned , there is no heat transfer, the combustion is perfect with every molecule of air finding a fuel molecule, and ignoring some extreme temperature effects. For simply comparing the actual fuel properties to one another, these factors are niggling.
As you point out the piston engine is basically an air pump. Each stroke it tries to pull in a fixed volume of fuel-air mixture to trap. Of course, there are a lot of factors that come into play on just how much air it does trap on each stroke, but for our purposes I think a comparison of the energy in the air-fuel mixture based on identical volume is the only rational basis. That is why I used the energy of a fixed volume to compare.
In the table, note that even though the ideal air/fuel mixture is 8.95 versus 14.88 in the table, the mass of air in the volume is actually only 5% less for the ethanol than gasoline. 58% more weight of ethanol was put in the mixture which displaced only 5% of the air because there is a lot more air than fuel. On a weight basis the ethanol only has 63% as much combustion energy as the gasoline, but 58% more ethanol was added, and the chemical balance of the equation works because the ethanol carries an extra oxygen molecule with it. This is for a mixture already vaporized. Basically in a carburetor it would mean jetting for a richer mixture but the engine is still pumping the same volume.
In a real engine, ethanol’s heat of vaporization is higher, cooling the mixture more as it vaporizes from liquid, theoretically dropping the temperature in the manifold and increasing density and the mass of mixture drawn in with each stroke. This would increase power a bit, if it happened that way. I have a 1920 SAE report on what it took to get the best power and fuel economy using pure ethanol in a tractor engine, and basically they had trouble vaporizing the ethanol and had to add more manifold heat. They also had to increase compression ratio (as you noted) due to the higher ignition temperature. They raised it to around 5/1 (stated as a compression pressure rather than ratio). In the end they got very good results with comparable efficiency after a lot of cut and try experiments. It included adding transparent windows in the intake manifold to observe un-vaporized liquid fuel running down the branches of the manifold. That was on pure ethanol. With only 10% ethanol in a gasoline blend that still has the more volatile gasoline components to light things off initially, I don’t know how much of a factor higher compression ratio and the higher ignition temperature is.
I can understand that 1920’s and earlier engines could have a tougher time on ethanol blends. With the very large combustion chamber and little turbulence the mixture is not going to finish mixing very well, having rich and lean spots, thus to insure combustion every time without misfire needs a rich mixture to make sure an ignitable mixture is at the spark plug. When it does burn it may burn slower and less complete due to the non-uniformity, even though ethanol itself has a higher flame speed. The mixture distribution to each cylinder from the manifold isn’t very uniform to start with compounding the problem. Besides higher compression ratio, a later 1930’s engine with Ricardo high turbulence “squish†chamber at least will better mix up the fuel and air near top dead center just before and during combustion. The ethanol may screw up the mixture distribution at least in part due to it cooling it a bit more and inhibiting vaporization. Ironically it might take more manifold heat to improve the situation, giving more vapor lock problems. There are potentially some other solutions, but they would require cut and try.
Even when running richer than stoichiometric a real engine will not consume 100% of the available oxygen and will leave some in the exhaust that may burn while traveling out to the tailpipe, much as air pumps were added in the ‘70’s to finish burning rich mixtures.
It would take a pretty extensive test cell and instrumentation setup to run these things to ground on any given engine, which is probably not in the cards.
Jim
I hope that Bill has gotten the basic answers to his original question before going further down the rabbit hole.
Ed, I am curious how you come to the conclusion that “Energy content can be broken down into energy and heat content, and the E10 has more heat and less energy.†Analysis of the thermodynamic properties of the combustion energy and products of combustion do not support this. Was this from observing the reduction in maximum power combined with an increase in measured exhaust gas temperature? If so, I assume the EGT was measured at the tailpipe with the gas analysis probe?
Just trying to untangle this to better understand what is going on.
The table shows the basic thermodynamic properties of the combustion products of pure ethanol and gasoline under perfect conditions for a fixed volume of mixture. The fundamental properties -specific heat coefficients, ratio of specific heat coefficients, and temperature rise with combustion – are remarkably close to each other.
This tells me that it is not the basic properties of combustion products that are causing the problems but the myriad other things going on in the engine that of course deviate from the ideal.
I suspect that the problems stem more from the differences in the blends at the carburetor and intake manifold before combustion. A reduction in power with an increase in exhaust gas temperature is probably related to slower and/or incomplete combustion coming from the fuel blend changing the atomization of the fuel in the intake manifold and messing up the fuel/air distribution to each cylinder. The higher heat of vaporization of ethanol dropping the temperature of the mixture while passing through the intake manifold is probably one factor. Ethanol does have a higher autoignition temperature that could come into play. Slower combustion from poor mixing can reduce power with an increase in exhaust gas temperature and incomplete combustion can keep burning in the exhaust manifold after leaving the cylinder – increasing temperature without adding power.
Perhaps this seems moot, but if we need to operate on current fuels it helps to try and understand what is going on.
Jim
Lead is only needed on pre-war engines to prevent exhaust valve seat recession, and the contemporaneous engineering literature indicates this did not become an issue until the 1930’s. It is caused by a combination of high exhaust gas temperatures, RPM and lean air fuel ratios. Even though the lower compression ratio’s of 1910’s era cars start out theoretically to have higher exhaust temperature than 1930’s, the lower RPM and high surface area’s of the combustion chamber quenches the mixture more by the time the exhaust valve opens. Another factor was likely the relatively poor fuel vaporization leading to less complete combustion. Liquid fuel in a rich mixture that doesn’t burn completely will quench temperatures and combustion. It also reduces the free oxygen that induces valve seat recession. Valve recession should not be an issue for 1920’s and earlier cars, and should not need special efforts to get 100LL aviation fuel or other additives.
The original purpose of lead in fuel had nothing to do with valve seat recession, it was to raise the octane and prevent knock, allowing higher compression ratios. Its benefits to valve seat life was not understood before WWII and played no role in whether an engine was “designed†to run on unleaded. As Tony points out, regular gasoline in 1925 only had an average octane rating of 55, premium was 71. It had risen to 72 and 77 respectively by 1935. An antique engine running 87 octane now has a serious problem if it exhibits any knock (ping). One of the problems can be buildups of carbon deposits in the combustion chamber from running too rich or oil blowby (or adding extra oil to the gasoline). The deposits can glow and cause pre-ignition. Early piston rings had poor oil control and typically were “de-carbonized†by removing the head at 15000 miles or so. If you have an engine that “Diesels” – keeps trying to run after ignition is cut – it is a sign of excess deposits.
Another factor is spark timing. A retarded spark increases exhaust gas temperature. This caused lots of heat problems in California in the ‘70’s and ‘80’s when many 60’s to 70’s cars were forced to have spark retarding devices to reduce hydrocarbon emissions. An antique in fine fettle running on 87 octane that was designed to run on 55 or 70 octane should be well clear of any knock issues and the spark can be advanced 5 degrees or so from original factory.
I think alcohol’s and modern fuel blends influence is mis-understood. There actually is very little difference in the energy content of the wide range of hydrocarbon constituents used to blend gasolines then or now. With perfect stoichiometric combustion of a vaporized mixture (not lean, not rich) ethanol actually has a tiny fraction (.1%) less energy per volume of mixture than gasoline. At the heavy end kerosene is about 2% less than gasoline, and at the lighter end butane is 1% less than gasoline. Butane is typical of the lightest (volatile) end of the many constituents of gasoline that evaporates very easily at low temperatures and is needed to start a cold engine. Ethanol does lean out a carburetor jetted for a rich mixture and run closer to stoichiometric, so it makes sense to adjust the mixture a bit richer, but remember the ethanol is only 10% or less to start with. A well controlled carbureted engine test done in 1941 showed a 10% ethanol blend increased net power by about 3 ½% after increasing the compression ratio slightly from 6.0 to 6.2 to obtain the same knock limit.
I think the main difference is not the basic energy content of the fuel but its volatility – its ability to atomize and evaporate in the carburetor and manifold to form a combustible mixture. Modern fuels in general tend to be a bit more volatile and evaporate/atomize more completely and this can increase power and temperature a bit (and creates the vapor lock problems). This is true of both conventional gasoline and ethanol. If the fuel doesn’t have ethanol it still must have hydrocarbon constituents in the same general range of boiling temperature as the 10% ethanol. Historical data shows that fuel volatility increased steadily from 1946 to 1965, well before ethanol was a factor.
I’m not defending ethanol, just saying it seems to be the go-to boogie man for all manner of engine problems rightly or wrongly.
Jim
Bill, I don’t have any experience with these older cars, and hope one of the members with experience on them will weigh in.
Presumably the mixture should be set to rich (heavy) for starting and warming up and leaned out for driving.
Speaking just in general terms for most any engine, a lean mixture will improve fuel economy for cruising down the road, will burn cleaner leaving fewer deposits and less spark plug fouling, but won’t develop as much power for climbing hills at wide open throttle and as you know will increase exhaust temperatures, be more susceptible to exhaust valve recession and knock (ping). However, I don’t think these early engines should be at any risk of that using current 87 octane fuel.
Jim
My local brake shop – which has a lot of experience with antiques – usually assumes the woven material is used for relining antiques. I think it is softer and takes less force than the later molded linings that are used on cars with higher performance brakes such as the ’33-38’s with power assist.
Jim
Exhaust manifold temp over 450 is fairly low, I have measured my ’35 845 at over 600F standing still. It will get hotter than that when being driven on the road under load. The exhaust gases flowing fast through the inside of the manifold will average on the order of 1000F, the manifold can only be cooled below that temperature by the slow 140 degree air coming out of the radiator wafting over the outside of the manifold and by conduction into the 200 degree block.
Note that high performance engines running at high power will make the exhaust manifold glow cherry red – ~1200F, but the block will be kept in the 200’s by the cooling.
The reason exhaust manifolds are susceptible to cracking is because of the large temperature difference between the manifold and the block they are attached too.
The manifold sits at the same temperature as the block until the engine is run, then quickly gets a lot hotter and tries to grow from thermal expansion which the connection to the cooler block prevents. This puts a strain on the manifold that may eventually crack it.
It looks like you have a good 20 degree temperature drop through the radiator and the block is being cooled down normally to a 200 degree temp via the flow of water starting at 150 and regaining the 20 degrees when it goes back to the radiator.
Jim
