- Home
- About Us
- Join/Renew
- Member Benefits
- Member Pages
- Log In
- Help
- Museum Store
Thanks Ed, got it!
Jim
Thanks Ed, maybe you could just email it?
Basic wire connections. It has 4 wires coming out of the box, + and – input, +/- output. No common ground. The converter case is not grounded, but you have to be careful if the case of the device you are powering is metal and grounded negative. It must be isolated from touching any metal in the car. My old Ipod case is metal and negative ground.Jim
The brake clutch facing turns with the driveshaft between the two pressure plates. Depressing the brake pedal presses the pressure plates which places a torque on clutch face. Inside there is a worm/spline arrangement that will push the main brake shaft via the oxbow shaft in the direction to apply the brakes whether the torque on the driveshaft is either forward or reverse. It is an interesting system since the brake power assist is the same fwd or reverse instead of relying on slight wind-in movement in the brakeshoes to help apply the brakes as in a conventional system, which is why most drum brakes require more pedal pressure in reverse than forward.
It is the torque being applied via the driveshaft that actuates the brakes, not the rotation of the driveshaft per se, except that which is required to take up all the mechanical slop in the system so it can still apply the brakes when stationary. The friction coefficient between the soft clutch lining material intentionally wet with transmission oil and the pressure plates of course a critical element in how the system operates, and undoubtedly the source of so much woe over the years. I am sure others with more experience will weigh in here.
I have added pictures of mine when I disassembled that shows the clutch lining material and pressure plates. I believe mine is original.
Jim

Here are measurements I took off the spears I was lucky to swap for my ’35.
Jim

Bill. Here are some pictures I took in 2003 of my 1935 845 before I disassembled. I have many more, thanks to one of the great boons to restoration – digital cameras. I can forward some via email if needed.
I think your 1933 should be about the same. Yes, Number 6 attached to the clutch pedal it is involved in the free wheeling operation. Note the springs involved in the connection. Don’t remember how it all works but was obvious when working the unit when reassembling. Yes, number 5 attaches to the freewheeling cable control.
The brake unit is an interesting piece!
Jim

You can find 1/2 inch OD steel or SS tube stock at McMaster Carr fairly reasonable with quick shipping.
Jim
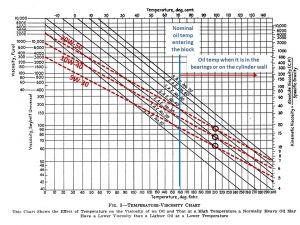
David, in your research I would be interested if you run into any data on the viscosity of multi-grades at elevated temperatures – 300 to 500 degrees. I thought I found some a few years ago but now can’t seem to find it.
If one takes the basic definitions of multi grade such as 10W-40, it has the same viscosity as SAE 10W at 0 degrees F and the same as SAE 40 at 210 deg F. This is shown on the chart – note that these charts are highly non-Cartesian on the y axis – straight 30 weight is about 50 times less viscous at 210 F as it is at 60F. Extrapolating this out would indicate that 5W-30 would have higher viscosity at 300 degrees than SAE 40 straight weight. This is relevant because the temperature of the oil film at top dead center will be closer to the temperature of the inside surface of cylinder wall at the top of ring travel rather than the bulk temperature of the oil in the pan when it enters the oil pump. This is greater than 300 degrees and a 5W-30 would actually be thicker at TDC and reduce the metal-to-metal boundary lubrication that cause the upper cylinder wall and compression ring to wear (tapering the bore). Note that ’30’s Pierce recommended nothing thicker than straight 30 weight in summer. This also holds true for the bearings – the local temperature of the oil film in the bearing will be hotter due to the local friction than the bulk oil temperature. meanwhile most of the oil in less critical areas will be less viscous, creating less heat and friction – an advantage of multi-vis.
The question though, is that it may be misleading to assume the oil viscosities will extrapolate above 210 as I show in the chart, so if you run into any data for elevated temperatures I would appreciate any links to it.
Jim
Ryan says the end play is when rotated, which I interpret to mean not in and out end play. Is it not normal to be able to rotate the tie rod slightly when the end play is correctly adjusted? I would think if it were so tight you couldn’t rotate it, it is way too tight and will increase steering effort and gall the ball joint. Maybe I maybe I am misinterpreting here.
Jim
Some notes on the Geo. Borg electric clocks, I have finished opening mine up, cleaning and lubricating. I made a new facia for the front clock and transplanted the rewind solenoid coil from the ’36 rear clock I obtained years ago. The ’36 rear also donated its beveled lens and facia to the rear ’35 clock. Both are running and keeping time.
Some notes for anyone else interested. I experimented with zener diodes, zener diodes controlling a transistor, and the slow blow fuse to try and cut the power to the clock when the voltage drops before the contact points started buzzing. None of them worked well. It also makes sense that there is enough wear on these clocks even when cleaned and lubed that they shouldn’t be left running continuously for long periods and a switch to turn them off or a master disconnect for the whole electric system would be best when the car sits.
It seems innate resistance of all of the protective electronic elements tried is too great. I measured the internal resistance of the rewind coil at 1.5 ohms, which means steady state the current would be about 4 amps. The internal resistance about 1 ohm more or less of the zener diode and MOSFET transistor was high enough to keep the rewind from working properly. The coil only snaps for less than a second, so a slow blow fuse of 1 amp did not blow despite the high instantaneous current. The resistance of a slow blow 500 milliamp fuse was too much and prevented the coil from getting enough current to rewind and separate the contact points, so it just let current pass continuously at least before I disconnected it.
The original fuse was 5 amps and would only protect for an outright short. I am going to use a 1 amp slow blow in mine. It won’t keep the contacts from buzzing and burning but might prevent the coil wire ends and solder joints from melting.
The rewind would work as low as 3 volts, started to buzz and get inconsistent at 2.75 volts, so the battery has to be pretty flat before it stops working and starts to buzz and burn the points.
Jim
Bill, do you have some experience with small job tiny quantity laser cutting, particularly cost? I have cogitated on making some new instrument parts and thought about getting them cut out via a CAD design file.
Jim
Jim, that is a good idea, it might need 4 to 5 amp as I measured the resistance of the coil at 1.5 ohms which would put the steady state current at 4-5 amps. It occurred to me that the zener might have a problem letting the contacts buzz as the inrush current to the coil drops the voltage. I was also thinking about adding a capacitor+resistor suppressor to keep the contacts from arcing.
I don’t want chords strung all over the garage to keep all the cars plugged in. I’d be tripping on the extension chords just getting around the garage. I’ve got enough stupid injuries. One car is always on the lift and easy to get messed up raising, lowering, and swapping. I do try to drive them often enough that it isn’t a problem, and batteries going flat isn’t that common a problem for me, I just screw up once every two years or so. The clocks are supposed to have their own fuse, probably for this reason, but the evidence seems to be they aren’t adequate protection for this problem. I don’t know at the moment how much voltage it takes to get the coil to open the contacts, but if one is out on tour with a weak battery that lets the thing start buzzing it could drain the battery in a hurry.
The zener seems like a very simple fail-safe with or without a trickle charger. Its a little disconcerting from a fire standpoint to watch those breaker contacts arcing and sparking continuously and realizing the wires get hot enough to melt the solder. The solder joint melted on one of mine was a big glob, not a tiny spot on a 22 gauge wire. The good news on these Pierce clocks is that they are contained in a metal case – unlike the cheesy plastic of later years-so unlikely it would start a fire, but it does fry the contacts and wires.
Jim, very sorry you are leaving. You have been a very big help to me with your very practical hands-on knowledge.
Thank you!
All the best! Jim
Sometimes it is interesting to wonder what happened to something along the way. The front clock mechanism looks to be in very condition, and just rewinding the main spring manually it took right off working. But…I couldn’t figure out how the electrical rewind was supposed to work. So I disassembled the rear seat clock and suddenly all was clear, the front clock was supposed to have a magnetic coil but someone had removed it. Why they removed it (to fix another clock?) and then reassembled it and put back in the car is a mystery. Maybe they thought they could find or wind a replacement coil and gave up.
The rear clock mechanism is the same basic design as the front but not the detail design. Years ago I bought a ’36 rear clock just for the beveled shaped crystal which is the same for ’35 rear clocks. Oddly, the ’36 rear clock work mechanism is 97% interchangeable with the ’35 front, but not the ’35 rear. Fortunately it has an intact coil. Looks like some open heart surgery.
Well, maybe the kroil did its work. When I slid the puller away, I slid my fingernail under the minute hand and it finally popped off. Hour hand as well – it was on brass. I now have the clock out of its case and it looks like new inside. The contacts for the electric rewind were closed, so first thing to check out is to see if they burned when the battery discharged enough to where it wouldn’t pull the spring back.
Jim
It is pretty hard to tell if the available clock arm pullers are the right scale for this particular clock, with flanges thin enough to get in between the minute and hour hands, and then strong enough to force them off. There is enough of the shaft sitting proud of the minute hand to make a miniature puller with a screw to push directly on the shaft instead of just trying to pull it off as shown. I am going to do that today. Thanks, Jim
Interesting idea, just brushing the whole eraser across the surface instead of trying to meticulously keep on the raised surface with a fine tip?
Thanks, Jim
Well, certainly is a complicated subject, with lots of opinions on the internet. Electrical not my thing. Apparently the DE-OX is a dielectric grease that one would think was an insulator but apparently does not increase resistance in a connection but rather keeps it from corroding, so I am guessing it won’t improve cleaned terminals initially but keep them from deteriorating over time and exposure. It is used on a lot of connections including battery terminals to keep them from oxidizing.
Many articles saying conductive greases aren’t, that they don’t actually improve connections and can cause corrosion problems if the wrong filler (carbon, silver, zinc) is used. Never the less there seem to be plenty of them out there.
The Stabilant 22 itself looks like the very amazing magic but expensive bullet.
It claims it can replicate the connection of a soldered joint, while amazingly not conduct electricity outside. Looks like it is a pretty big deal in the world of computers and audiophiles. I got very confused because the SL-5 was listed as a silicone dielectric grease for protecting spark plug connections but also shows up when searching Stabilant 22. It appears Standard Ignition products sells the SL-5 as a kit including the silicone dielectric and a tiny bottle of Stabilant 22 as well.
Jim
Thanks, I’ll check them out. Jim
